Expert Talk: Can plasmon catalysis turn the spotlight on CO2 conversion?
Emissions of carbon dioxide are considered to be the main cause of climate change. To achieve the necessary emission reductions, measures need to be taken in various areas, by concentrating on renewable energy sources, energy efficiency and recycling of materials. In this context, Carbon Capture and Utilisation (CCU) will play an important role.
Written by Ken Elen, senior researcher at imec/UHasselt/EnergyVille. Dr. Ken Elen received a BSc in chemistry from LUC in 2003 and graduated as MSc in chemistry at the KULeuven in 2005. After obtaining his PhD in inorganic and physical chemistry (UHasselt, 2010), he was employed in the inorganic and physical chemistry group (previously IPC, now DESINe) of IMEC-IMOMEC and as research associate of Hasselt University. His current research focuses primarily on the synthesis and application of ceramic nanomaterials and coatings in the field of optics and electronics (e.g. oxide semiconductors, TCO’s, (photo)catalysts, etc.) by building up generic knowledge in precursor formulation for metals and metal oxides, film formation through wet deposition, thermal processing, and their influence on the materials performance. At present, Ken Elen is (co-)author of 25+ publications in this field.
CCU covers a variety of processes in which CO2 is collected from point sources or by direct air capture, and used to synthesise new molecules with an added economic value, which will require a high amount of renewable energy. These molecules can either be introduced into the natural gas grid in the form of synthetic methane (Power-to-Gas), can be used for mobility purposes (Power-to-Mobility, given the volume restriction most probably liquid) or can be used as feedstock for the chemical sector (Power-to-Chemicals). Utilisation of CO2 can be realised by a variety of complementary technologies, such as (photo-)electrochemical, biochemical (e.g. micro-algae), and plasma-assisted processes. This Expert Talk will give a brief overview of methods that rely on the catalytic conversion of CO2, with dedicated attention to recent developments that allow these processes to be carried out using sunlight as a sustainable energy source.
Conventional methods for the catalytic conversion of CO2
Catalytic conversion of CO2 involves the reduction reaction of carbon dioxide at elevated temperatures using heterogeneous catalysts. Many of these methods were discovered in the early part of the 20th century and some have since been adopted in various industrial processes. The technologies with the highest readiness level for the catalytic conversion of CO2 to synthetic fuels or their precursors (i.e. syngas) are methanation by the Sabatier process, the Reverse Water-Gas Shift (RWGS) reaction, and Dry Reforming of Methane (DRM). Subsequently, syngas can be converted further into various (oxygenated) hydrocarbons using the Fischer-Tropsch process.
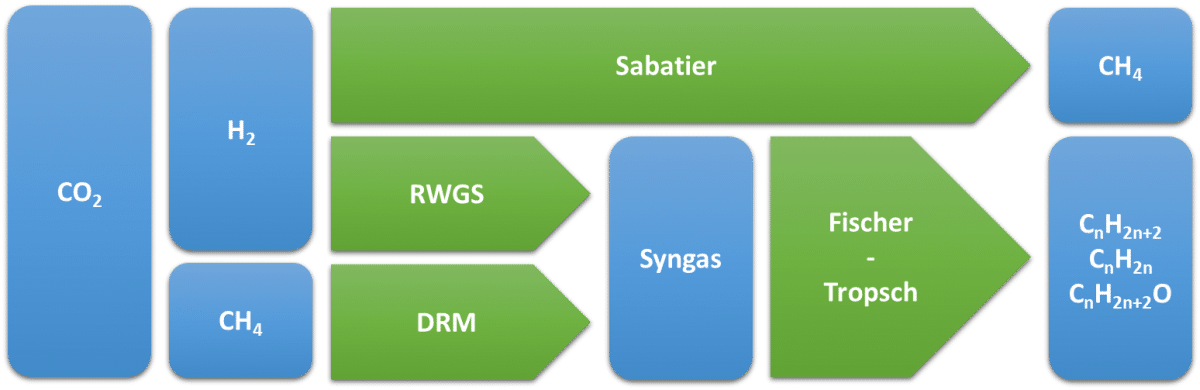
Sabatier reaction
During methanation of CO2, also known as the Sabatier reaction, carbon dioxide is reduced with hydrogen to produce methane, a frequently used fuel for heating homes or generating electricity.
CO2 + 4 H2 ⇌ CH4 + 2 H2O
Conventionally this is achieved using a heterogeneous catalyst (mostly nickel) at elevated temperatures (400-500°C) and pressures up to 100 bar. The ability to generate synthetic methane from carbon dioxide provides the prospect for recycling carbon as a fuel. As a result, it is considered as an advantageous technology for the mitigation of CO2 emissions. The reaction can be optimised towards an overall CO2 conversion of 100% into synthetic methane, with an electricity requirement of approximately 15 MWh/t. At this moment, the Sabatier process is already operated at a pilot and semi-industrial scale in Europe. For example, as of 2013 Audi runs a facility in Germany that produces synthetic methane from CO2 and renewable hydrogen.
Reverse water-gas shift reaction
By altering the catalyst and the ratio between CO2 and H2 the conversion of CO2 can be redirected towards the production of carbon monoxide in a reaction known as the reverse water-gas shift.
CO2 + H2 ⇌ CO + H2O
The RWGS reaction is endothermic, and has a low equilibrium constant, even at high temperature. Typical process temperatures are between 500°C and 800°C. Because of the low equilibrium constant, the process must be fed with either H2 or CO2 rich mixtures for satisfying results. Typical CO2 conversions are around 50% for reactions carried out at 500°C, while temperatures up to 800 °C are required to achieve a 75% conversion. Conventional catalysts for this process are Cu, Pt and Rh immobilised on a variety of supports. As carbon monoxide is the main reaction product, the RWGS reaction is considered as a potential route for the renewable production of syngas, a mixture of CO and H2 which can be used as a feedstock for the subsequent production of higher hydrocarbons using the Fischer-Tropsch process (see further on).
Dry reforming of methane
A different sustainable syngas production method can be provided by dry reforming of methane. This process refers to the direct formation of syngas by the reaction between CO2 and methane.
CO2 + CH4 ⇌ 2 CO + 2 H2
Commonly reported operating conditions state temperatures of 800-900°C in combination with a heterogeneous catalysts such as nickel or cobalt or noble metals such as rhodium or platinum. Despite its capability in converting greenhouse gasses, DRM is not yet widely-used in the gas processing industries because of rapid catalyst deactivation due to carbon deposition. Nonetheless DRM has the prospect for economic and ecological benefits, as the feedstock for the reaction (CO2 and CH4) is fairly inexpensive and does not rely on the supply of (green) hydrogen.
Fischer-Tropsch process
Fischer-Tropsch (FT) comprises the reactions used to convert syngas into alkanes and alkenes of longer chain length. At the same time, other products are produced, such as alcohols or aromatic compounds, but in much smaller quantities. The Fischer-Tropsch process is typically used in the production of synthetic diesel fuel or fine chemicals. Even though FT processing does not consume CO2 directly, it is used frequently in combination with the RWGS or DRM to convert renewable syngas into value-added hydrocarbon compounds.
n CO + (2n+1) H2 ⇌ CnH2n+2 + n H2O
n CO + 2n H2 ⇌ CnH2n + n H2O
n CO + 2n H2 ⇌ CnH2n+2O + (n-1) H2O
Typical operating temperatures range between 200 and 350°C, with lower temperatures showing a higher production of liquid fuels. A major concern with the FT process is the parallel water-gas shift (WGS) reaction, by which CO is again converted to CO2. The extent of the WGS reaction can however be minimised using cobalt-based catalysts.
From heat to light-assisted conversion of CO2
As illustrated above, conventional processes for the catalytic conversion of CO2 require intense heat as source of energy to drive the reactions. Instead, direct use of sunlight as energy source would be an appealing alternative. To this end, semiconductor metal oxide photocatalysts have risen to the attention. Owing to their bandgap they use only a small part of the solar spectrum and consequently require high-intensity UV light. The UV part of the solar spectrum comprises only 4% of the total sunlight. This directly translates into low space-time-yields (STY) for such catalytic processes. For solar water splitting, the STY is approximately 1 kg m-2 year-1, and for CO2 conversion even lower. The shortcomings associated with semiconducting photocatalysts can be mitigated by exploiting the unique optical properties of metallic nanocatalysts.
Nanoplasmonics and plasmon catalysis
Metallic nanoparticles exhibit the ability to enhance the interaction between light and matter. Upon illumination the free electrons of such nanoparticles will start to resonate with the oscillating electro-magnetic field. These resonant electronic oscillations are known as localised surface plasmons (LSPs). Nanoplasmonics is the scientific field that studies the fundamentals and applications of LSPs. Due to excitation of LSPs, light absorption and scattering by metallic nanoparticles can be enhanced significantly, often leading to vibrant colours. Although the physical background behind this phenomenon was unidentified for centuries, its effect has been used historically in the fabrication of stained-glass windows. By the advent of the Mie theory, it is now understood that the spectral behaviour of this resonance depends on the geometry of the nanoparticles, the properties of their constitutive material, and their environment. These insights have contributed to the current status of nanoplasmonics, which has grown into a highly developed and advanced research topic with applications ranging from photovoltaic systems to biomedical theranostics. Within the context of catalysis, nanoplasmonics can be implemented to enhance the efficiency and selectivity of chemical reactions: in addition to unique spectral properties, LSPs are accompanied by valuable physical effects such as optical near-field enhancement, excitation of electrons, and heat generation.
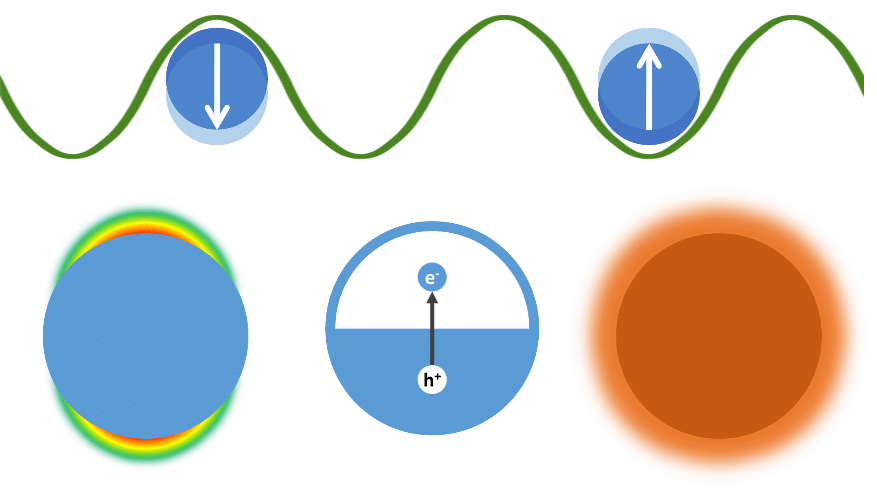
Plasmoncatalytic conversion of CO2
Since illuminated plasmonic nanoparticles can behave as efficient sources of heat and/or energetic electrons, nanoplasmonics offers a convenient approach for activating chemical reactions by light. Additionally, using a mixture of different sizes and shapes, plasmon catalysts can easily cover the entire solar spectrum, enabling the use of all sunlight for the chemical process of choice. As an example, the Buskens group (TNO, The Netherlands) and the DESINe group (UHasselt/Imec and partner in EnergyVille) have conjointly reported on plasmonic catalysts which broadly absorb sunlight for the light-powered Sabatier process and yield a STY of approx. 29 kg m-2 day-1 [ACS Omega 4 (2019) 7369-7377], i.e. 10,000 times more than the figure stated above for semiconducting photocatalysts. Based on a similar concept, the Halas group at Rice University (Houston, Texas) have recently demonstrated a light-stimulated plasmon catalyst that efficiently conducts the DRM process without additional input of heat [Nat Energy 5 (2020) 61–70].
These recent findings show that plasmon catalysts have a bright future in paving the way for a transition from a heat-driven to a (sun)light-driven conversion of CO2. Still challenges remain to bring this new technology to a competitive level. One intrinsic limitation is that powerful catalyst nanoparticles for CO2 conversion display only a weak plasmon resonance, typically positioned in the UV. Strong plasmonic metals however, do not catalyse the conversion of CO2 as effectively. An inspiring strategy is to combine the best of both in bimetallic or composite plasmon catalysts, where a strong plasmon resonator harvests the light from the sun and couples or transfers its energy to the catalytic particle. Another challenge will be the design of innovative reactor concepts that allows sunlight-powered chemical reactions on an industrial scale. To this end, comprehensive systems of transparent reactors, secondary solar optics, and/or energy efficient LED light sources to ensure continuous operation are under development. Through the combined effort of materials science and process engineering, plasmoncatalytic conversion of CO2 can be established as an effective process for the sunlight-assisted production of value-added chemicals.
Currently, the DESINe group actively participates in a number of research programs that should pave the way for the sunlight-assisted conversion of CO2. In the Moonshot-project “D2M” new bimetallic plasmon catalysts are being designed and synthesised to enhance the conversion of CO2 to syngas. Furthermore, in the Interreg-project “LUMEN” we aim to create an integrated, laboratory-scale demonstrator which can validate that sunlight-driven conversion of CO2 with hydrogen into methane and/or syngas is feasible from both a technical and economic perspective. The research within “LUMEN” provides the basis for the future translation into an industrial process and offers commercial opportunities for material and equipment producers and chemical companies.
Concluding remarks
Reducing carbon dioxide emissions and securing our future energy supply by switching from fossil fuels to sustainable energy sources are essential contributions for our transition towards a carbon neutral society. Catalytic conversion offers the prospect of large-scale recycling of CO2 emissions, but requires intense heat to drive the reactions. Both challenges can be tackled simultaneously using plasmon catalysis: CO2 can be converted to value-added chemicals, and this is achieved using sunlight as a sustainable energy source. Nonetheless, bringing this new technology to a competitive level will require a combined effort on many fronts, from of materials science to reactor design. Furthermore, since many of the conversion processes consume hydrogen, an affluent supply of green hydrogen will be indispensable to consider these technologies as truly renewable as well as economically viable. Ultimately, no single approach will emerge as the panacea to the detrimental effects of CO2 emissions. Instead, many of the complementary technologies, which are currently in development, will contribute conjointly to a sustainable future.
Acknowledgements
Project “LUMEN” is financed within the Interreg V program Flanders-The Netherlands, the cross-border cooperation program with financial support from the European Fund for Regional Development, the Dutch government, and the provinces of North-Brabant (NL), Limburg (NL and BE) and East Flanders (BE). More info: www.grensregio.eu
Project “D2M” is a cSBO project within the Moonshot research trajectory MOT-3 on electrification and radical process transformation with financial support from the Flemish Agency for Innovation and Entrepreneurship (VLAIO). More info: www.moonshotflanders.be



